Not actual size.
Up to 12-month shelf life at room temperature1,2†
| † | Vials may be held up to 12 months upon removal from refrigeration to room temperature storage conditions between 20°C to 25°C (68°F to 77°F), anytime within the labeled shelf life.1,2 |
24-month total shelf life1‡
| ‡ | Product requires refrigeration between 2°C to 8°C (36°F to 46°F); do not freeze.1,2 |
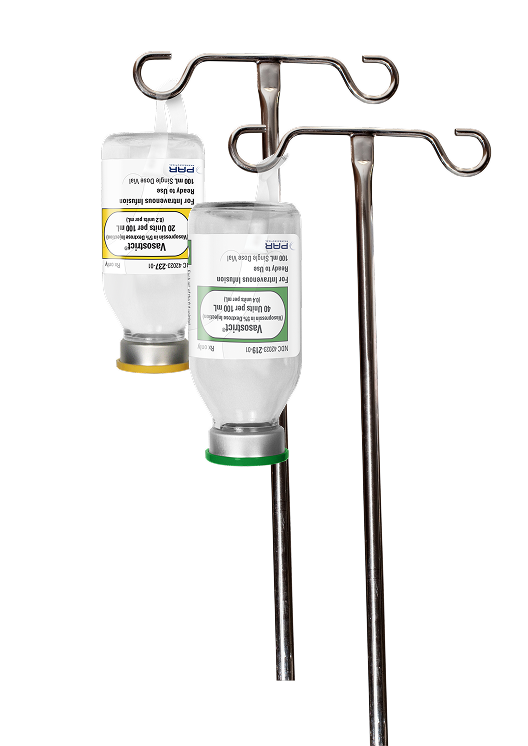
Vasostrict® is a sterile, aqueous solution of synthetic arginine vasopressin for intravenous administration.2 Vasopressin is a polypeptide hormone. Vasostrict® is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
Vasostrict® Premixed Solution comes in ready-to-use, single-dose vials that do not require further dilution prior to administration.2
| May offer enhanced inventory management with 24-month total shelf life1§ | |
| Preservative-free | |
| Available through your wholesaler | |
| Does not require compounding, diluting, mixing, or transferring, which may reduce waste3 and the risk of preparation error4 | |
| Compatible with most automated dispensing machines | |
| Aligns with medication strategies of ASHP Guidelines on Preventing Medication Errors in Hospitals4 |
| § | Product requires refrigeration between 2°C to 8°C (36°F to 46°F); do not freeze.1,2 |
References: 1. Data on file. DOF-VS-02. Endo USA, Inc.; September 21, 2023. 2. Vasostrict®. Prescribing Information. Endo USA, Inc. 3. American Society of Health-System Pharmacists. ASHP guidelines on medication cost management strategies for hospitals and health systems. Am J Health-Syst Pharm. 2008;65:1368-1384. 4. American Society of Health-System Pharmacists. ASHP guidelines on preventing medication errors in hospitals. Am J Health-Syst Pharm. 2018;75(19):1493-1517.
